Guidelines for Groundwater and Surface Water Sampling for Cations, Anions, Stable Isotopes, and Nutrients (N and P).
- Introduction
1.1 Purpose and Scope
To provide standardized methods for collecting, preserving, and transporting groundwater and surface water samples for the analysis of cations, anions, stable isotopes, and nutrients (N and P).
Designed for use by policymakers, regulatory bodies, environmental managers, and field technicians.
1.2 Importance of Accurate Sampling
Reliable water quality data supports effective resource management, pollution control, and regulatory compliance.
Consistent protocols ensure data comparability across regions and time.
 2. General Considerations
2. General Considerations
2.1 Site Selection and Planning
Choose representative sampling points considering hydrology, geology, land use, and pollution sources.
Clearly define sampling objectives (e.g., baseline monitoring, contamination assessment).
2.2 Health, Safety, and Environmental Protection
Ensure field teams follow safety protocols, including proper use of personal protective equipment (PPE).
Minimize disturbance to natural water systems and habitats
- Sampling Preparation
3.1 Equipment and Supplies
Use acid-washed HDPE, glass, or polypropylene bottles depending on analyte sensitivity.
Ensure all equipment is pre-cleaned and contaminant-free.
Use high-purity deionized water for rinsing if field filtration is required.

3.2 Quality Assurance and Control
Include field blanks, trip blanks, and duplicate samples.
Maintain accurate field logs and chain of custody records.
Physicochemical parameters should be measured in situ, which should be done after calibration of each equipment with the right standard.
- Sampling Procedures
4.1 Cations (e.g., Na⁺, K⁺, Ca²⁺, Mg²⁺, Fe²⁺)
Collect samples in acid-washed bottles to prevent contamination.
Filter to 0.45 µm immediately if necessary.
Acidify to pH <2 with ultrapure HNO₃ and store at 4°C, protected from light.
4.2 Anions (e.g., Cl⁻, NO₃⁻, SO₄²⁻, HCO₃⁻)
Use non-acidified, pre-cleaned bottles.
Filter to 0.45 µm if necessary, without acidification.
Store at 4°C to prevent microbial alteration.
4.3 Stable Isotopes (e.g., δ¹⁸O, δ²H, δ¹³C, δ¹⁵N)
Use airtight glass vials, ensuring no headspace to prevent evaporation. Store, in a dark, temperature-stable environment.
4.4 Nutrients (Nitrogen and Phosphorus)
Collect in opaque, pre-cleaned bottles to limit light exposure.
Filter to 0.45 µm if necessary. Acidify or store immediately at 4°C depending on the analyte.
- Sample Handling and Transport 5.1 Labeling and Documentation
Clearly label with sample ID, date, time, location, and preservation method.
5.2 Transport Conditions
Use coolers with ice packs to maintain low temperatures. Avoid direct sunlight and prolonged transit times.
- Laboratory Analysis and Data Management
6.1 Analytical Methods
Use standardized methods (e.g., ICP-OES, IC, MS, IRMS) for precise and accurate measurements.
6.2 Data Quality and Reporting
Use reference materials and quality control samples.
Document data quality, including detection limits and measurement uncertainty.
- Conclusion and Best Practices
Regularly review and update protocols based on technological advances and regulatory changes. Train field personnel to ensure consistency and accuracy. Ensure transparent data management for regulatory compliance and public trust.
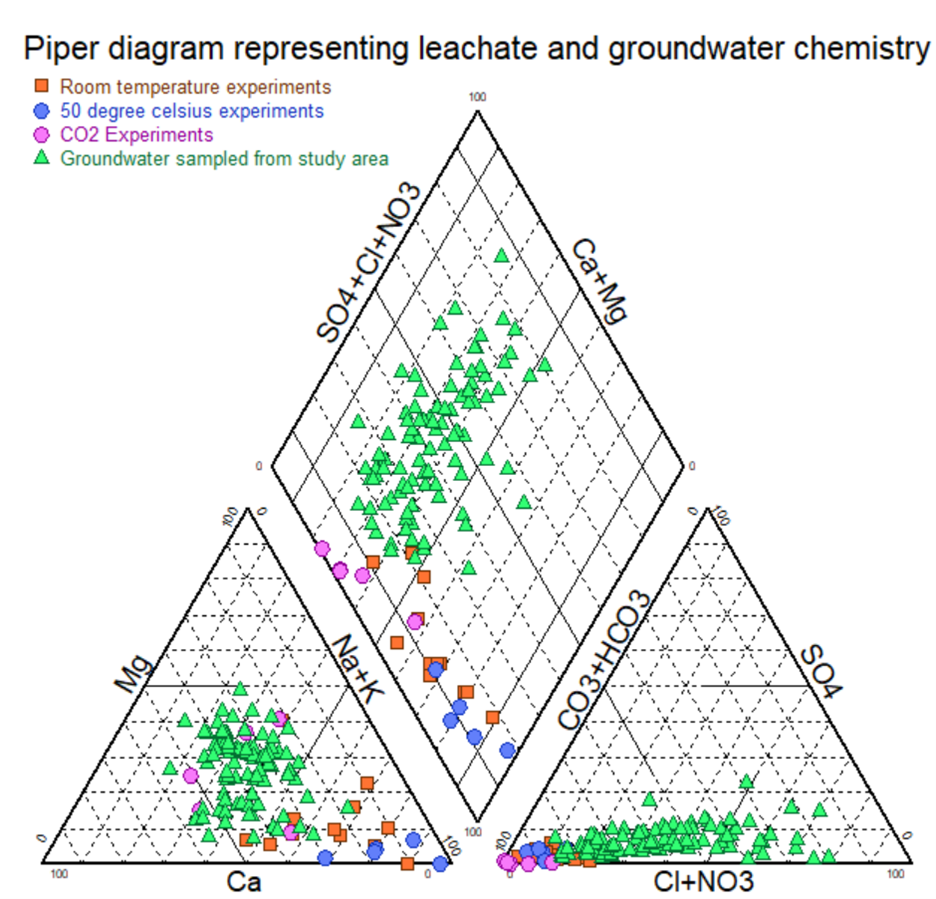
Tafadzwa Samantha Mutanga (Pictures attached to this article) is meticulously adhering to these set of protocols throughout her PhD research. This systematic approach has been instrumental in gathering high-quality data, which is essential for informed decision-making and the development of effective policies.