HOW DOES GROUNDWATER ACQUIRE ITS UNIQUE CHEMICAL SIGNATURE?
A team of scientists, led by Priscilla Lartsey, set out to explore an intriguing question: How does groundwater develop its distinct geochemical signature? To answer this, they conducted a fascinating and rigorous experiment at the Geotop laboratories at the Université du Québec à Montréal in Canada.
Before traveling to Canada, Priscilla and her research team in Ghana conducted a field campaign in the north-western part of the Volta River Basin, the site of her PhD research. During this campaign, they collected samples of five different silicate rocks from various geological units across the basin. These rock samples were then transported to the Geotop lab for controlled experimentation.
The main objective was to investigate how different environmental conditions influence the dissolution of minerals from rocks into groundwater — a key process in shaping its chemical signature. The team simulated four experimental conditions designed to mimic natural subsurface environments:
- Room temperature exposed to the atmosphere,
- Elevated temperature at 50ºC,
- CO₂-saturated environment (at 1 bar pressure),
- Acidic environment using nitric and sulfuric acids (HNO₃ & H₂SO₄).

Before the experiments began, the mineral composition of each rock sample was analysed using a petrographic microscope and X-ray Diffraction (XRD). The analysis revealed that the rocks consisted of common silicate minerals such as Quartz, Albite, K-Feldspar, Ca-Amphibole, Epidote, Biotite, Chlorite, and Muscovite.
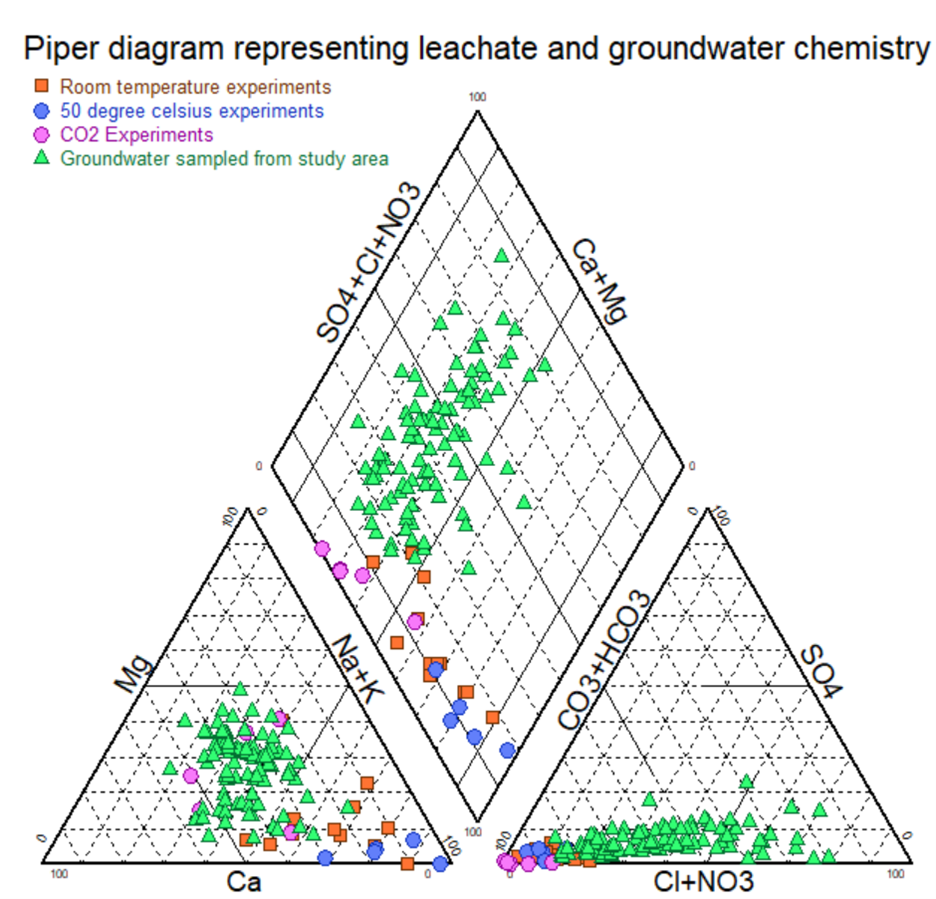
Throughout the experiments, the team monitored changes in electrical conductivity (EC) and pH of the leachates to track the progress of mineral dissolution. Results showed that variations in EC and pH were influenced by the mineral composition of the rocks, the duration of the reaction, and the experimental conditions.
Interestingly, the leachates from the CO₂-saturated condition closely matched the natural groundwater chemistry from the field site in Ghana. This finding highlights the crucial role of carbon dioxide in rock-water interactions. Further analysis through mass balance calculations confirmed that the primary contributors to the observed groundwater chemistry were the dissolution of minerals like Albite, K-Feldspar, Ca-Amphibole, and Biotite — releasing major ions such as Na⁺, K⁺, Ca²⁺, Mg²⁺, HCO₃⁻, Cl⁻, NO₃⁻, and SO₄²⁻.
So, what’s the big takeaway?
It turns out that rainwater, when mixed with natural CO₂ from the air and soil, becomes just acidic enough to dissolve rocks very slowly over time — and that’s how groundwater gets its unique mix of minerals. Pretty amazing, right?





